Although SiC is an atomic crystal, it is a semiconductor with good nonlinear conductivity, and its resistance decreases with the increase of the electric field.
1. The color of silicon carbide
Pure silicon carbide is a colorless and transparent crystal, and the reason for the color of silicon carbide is the incorporation of impurities. For example, boron carbide will make the crystal black, and the crystal will be green when nitrogenized. Silicon carbide abrasives classify colorless to green silicon carbide as green silicon carbide, and dark blue to black silicon carbide.
In addition to the color of the crystal itself, the surface film of silicon carbide crystal also has an interference effect on natural light. When the thickness of the silicon oxide film on the surface of the silicon carbide crystal is different or the angle of reflection of light is different, the reflected light is two-phase coherent light. Therefore, the surface of silicon carbide has a mottled, colorful color under natural light.
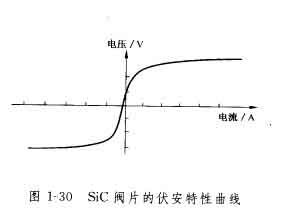
2. Silicon carbide is a semiconductor with a resistivity of 10-2~1012Between Ω·m, it varies with the type and amount of impurities in the crystal. The most influential impurities are aluminum, nitrogen and boron. When there is more aluminum, the conductivity of silicon carbide increases significantly. The conductivity of silicon carbide increases rapidly with the increase of electric field strength, and it has the characteristics of nonlinear change as shown in Figure 1-30, and V=C·I can be usedαDenote. where V is the voltage, C is the material constant, and α is the nonlinear exponent. The arrester valve is made using this semiconductor property of silicon carbide.
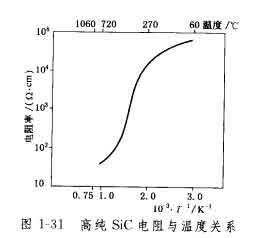
The resistivity of silicon carbide changes with temperature, and the resistivity becomes smaller with increasing temperature, which is opposite to the temperature characteristics of the metal, as shown in Figure 1-31.